Health Canada has released their Quarterly Compliance and Enforcement Report – Inspection Data Summary for cannabis inspections conducted between April 1, 2018, and March 31, 2019.
The last annual report was for fiscal year 2017-2018, provided in May 2019. Health Canada says they encountered delays in publishing the latest information due to the COVID-19 pandemic. Previous reports include inspection data for 2016-2017 and 2015-2016.
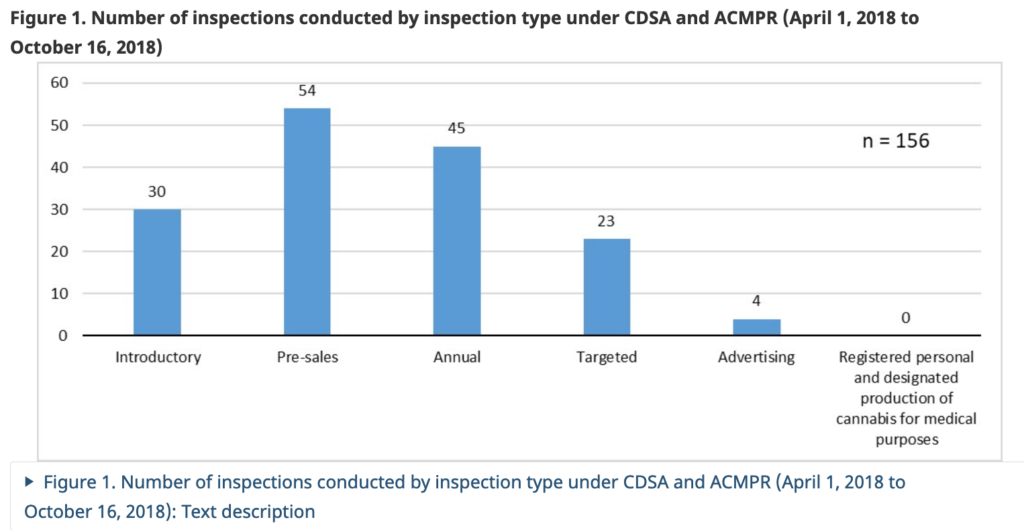
The types of inspections prior to October 17, 2018 under the CDSA, ACMPR, and FDA and after October 17, 2018 under the Cannabis Act and Cannabis Regulations are quite similar. The main difference is previous “pre-licence inspections” changed to sales inspections, annual inspections changed to regular inspections and advertising inspections changed to marketing inspections.
An observation is a deficiency or deviation from legislative or regulatory requirements that is noted by an inspector during an inspection, and confirmed in writing to the licence holder in the inspection report. Critical observations are those that are likely to: increase the immediate risk of diversion to prevent the detection of diversion, or present an imminent health risk, or may also involve the possibility of deliberate fraud, misinterpretation, or falsification of information.
An inspection can be assigned a compliant or non-compliant rating, based on the number and severity of observations noted during an inspection.
A total of 293 inspections were conducted from April 1, 2018 to March 31, 2019.
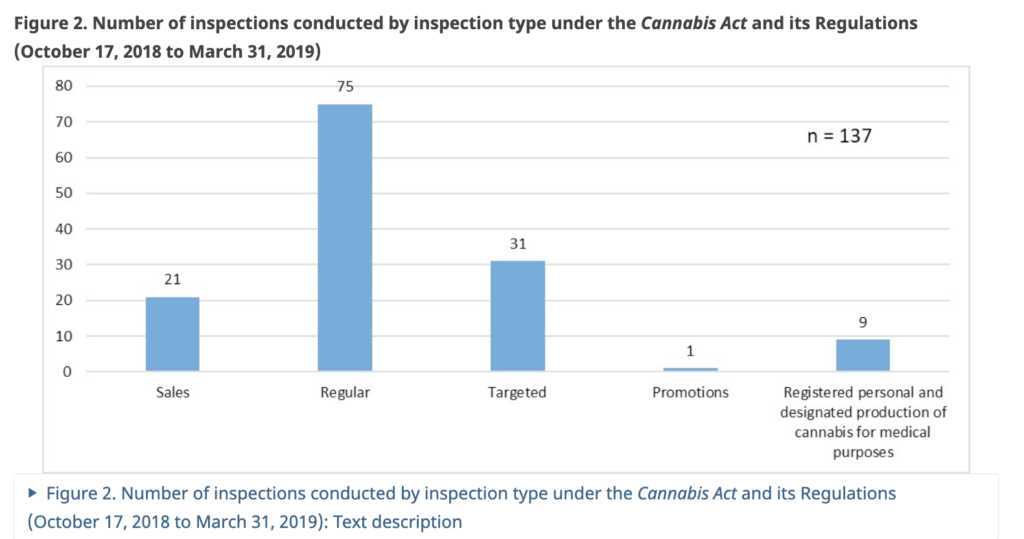
In the six months prior to legalization (April 1, 2018 to October 16, 2018), 156 inspections of regulated parties were conducted under the CDSA and the ACMPR. From October 17, 2018 to March 31, 2019, there were 137 inspections of regulated parties conducted under the Cannabis Act and its Regulations.
Of those 137 post-legalization inspections, 21 were for sales, 75 were regular, 31 were targeted, one was for promotions and nine were of registered personal and designation production of cannabis for medical purposes.
In the six months prior, there were no inspections of personal or designated medical producers.
Between April 1, 2018 and October 16, 2018, under CDSA and ACMPR, four warning letters were issued to formally advise licence holders of their non-compliance and to require corrective measures, one resulted in a seizure and detention of products was conducted and one licence was suspended (Agrima Botanicals).
Between October 17, 2018 and March 31, 2019, under the Cannabis Act and its Regulations, one warning letter was issued to formally advise the licence holder of their non-compliance and to require corrective measures, one seizure of products was conducted and one licence was suspended (Bonify).
Five inspections at promotional events were conducted from April 1, 2018 to March 31, 2019, two in Toronto, one in Oro-Medonte, ON, one in Victoria, BC, and one in Regina, SK.
In the six months after legalization, Health Canada conducted 85 promotions related compliance verification activities which resulted in 48 actions taken. These actions include compliance promotion letters or referrals to RCMP. During the reporting period, Health Canada undertook nine Compliance Promotion Calls, three Compliance Promotion Letters, four Warning Letters, and 32 Referrals to RCMP.
There were also 38 Compliance Promotion Calls, three Compliance Promotion Letters, and eight Compliance Promotion Sessions.
Health Canada also says it has taken several steps to strengthen its oversight of persons authorized to produce a limited amount of cannabis for medical purposes. This includes scrutiny of high gram amount authorizations and engagement with provincial and territorial colleges of physicians to look into these physicians activities, continued engagement with munis and law enforcement on how the medical access system works, as well as applying new powers to refuse or revoke a registration on the grounds of public health and public safety, and increasing the focus on compliance promotion with registrants.
Personal and medical authorization sites have been coming under increased scrutiny by several municipalities, as well as many Conservative MPs, especially in Ontario where OPP says criminal enterprises exploiting the Health Canada medical, personal and designate cannabis production regime.
A southern Ontario county says they are the first in Canada to take steps to manage personal and designated medical grow licenses through local zoning bylaws.
In March 2019, nine inspections associated with nine registrations were conducted at four authorized production sites in the province of Ontario, three in London, one in Markham, ON, nine observations were made in total for the nine inspections, one inspection resulted in no observations, and eight inspections resulted in one to two observations for each inspection.
As of June of this year, 33,614 were signed up to either grow their own cannabis or have it supplied by a designated grower.