Health Canada has released their newest compliance and enforcement report covering April 1, 2020 to March 31, 2021.
The newest update comes after the report for the previous year was released last February, and data for 2019 was released in December of last year.
Although in-person inspections were limited in 2020 and 2021 due to Covid-19 restrictions, on-site planned inspections were resumed in the fiscal year 2021-22.
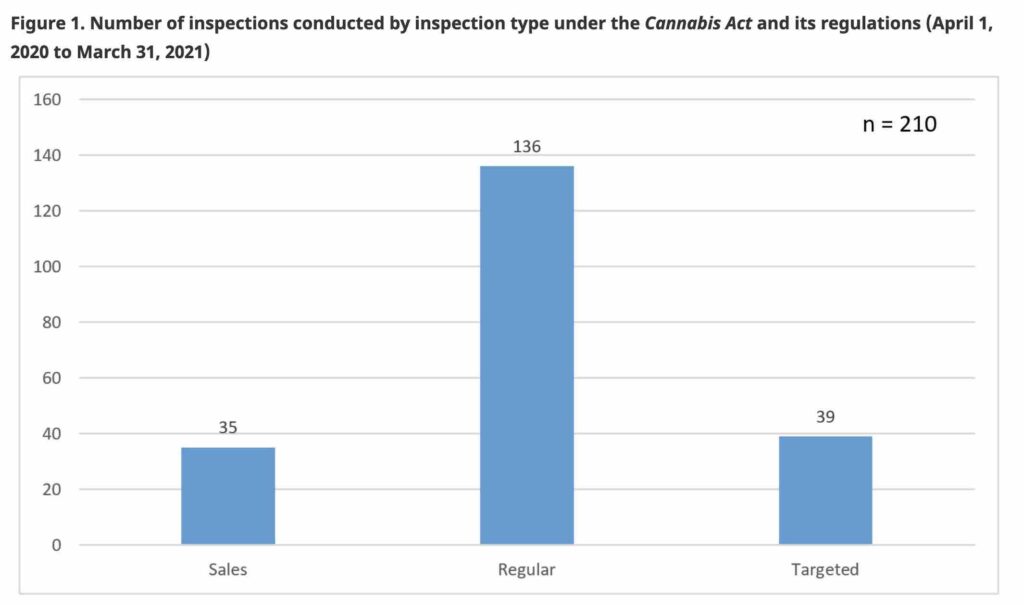
The newest figures cover 210 inspections of federally-licensed cannabis businesses across Canada. During this reporting period, Health Canada issued a total of 14 non-compliant inspection reports under the Cannabis Act and its regulations.
This compares to a total of 417 inspections of regulated parties that were conducted in the previous year (April 1, 2019 to March 31, 2020).
Issues observed in this most recent reporting period included: non-compliance with Good Production Practices (GPP), inadequate record keeping, inventory discrepancies, unauthorized activities, and failure to meet certain physical security requirements.
Of those 210 inspections, 136 were classified as regular inspections, 39 were targeted inspections, and another 35 were sales inspections.
Sales Inspections relate to the issuance of a licence amendment to authorize the sale of additional classes of cannabis products such as dried/fresh cannabis, edibles, extracts, and topicals.
The sales inspection seeks to verify that the licence holder meets the requirements of the federal Cannabis Act and Cannabis Regulations including, but not limited to, Good Production Practices (GPP), packaging, labelling, shipping, and record keeping.
Regular Inspections are undertaken to monitor and verify the licence holder’s compliance with the requirements of the Act and its regulations.
Targeted Inspections are to verify compliance of the licence holder with specific areas of the Act and its regulations.
In total, these inspections led to three warning letters being issued, two seizures and detentions of products, and one licence being suspended.
One BC producer, Ten-10 Ventures Inc., faced a regular inspection followed by two targeted inspections that led first to a notice of licence suspension and then to the seizure of cannabis due to security, good production practices, and record keeping.
Ten-10 Ventures was first listed as suspended on October 30, 2020, and was then listed as reinstated in June 2021.
In addition to these inspection activities, Health Canada also oversees compliance with federal promotional restrictions. In this same time period (April 1, 2020 to March 31, 2021), Health Canada conducted 294 “promotions-related compliance verification activities”.
These verification activities were carried out based on what the federal regulator says is “information received on alleged non-compliances with the promotional prohibitions under the Cannabis Act”.
This resulted in 85 enforcement actions such as warning letters, compliance emails, and compliance promotion emails or calls. These included two warning letters, 39 compliance emails or letters, and 44 compliance promotion emails or calls.
Health Canada notes that warning letters represent a high-priority action while a compliance promotion email or call represents the lowest.
Past annual reports have included inspection data on registered personal and designated production of cannabis for medical purposes. Between April 1, 2019 and March 31, 2020, there were 82 inspections associated with 82 of these types of registrations.
This newest report did not include information on these types of inspections, as on-site planned inspections—including inspections of registered personal and designated production of cannabis for medical purposes—were postponed in 2020 due to Covid-19. These on-site planned inspections were resumed in the fiscal year 2021-22 and are therefore not covered in this reporting period.
The full report, as well as past annual reports, are available here.