
Health Canada has released their first two reviews on Adverse Reaction Reports associated with cannabis products.
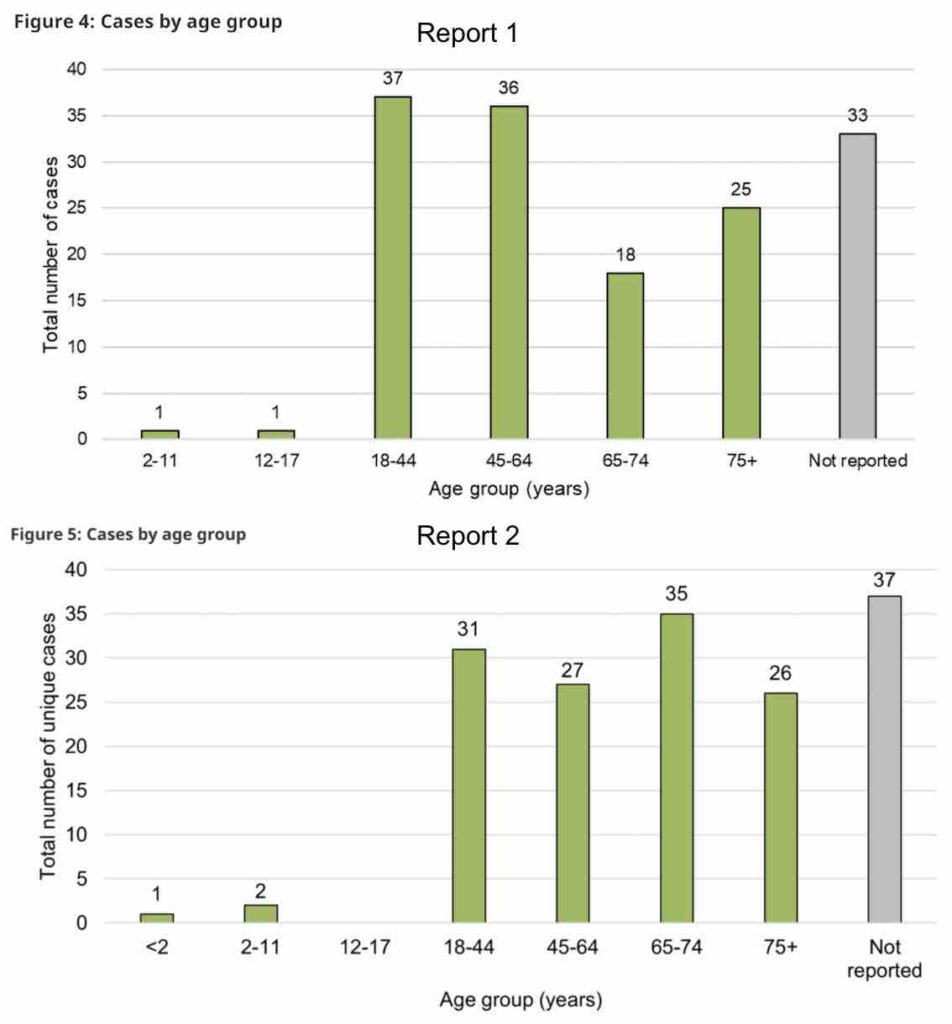
The most common reports of adverse reactions to cannabis involved ingestible cannabis oils or softgels, and reports were most often from adult women. Younger adults were more likely to report negative effects from inhalable forms of cannabis like dried flower or extracts, while older adults were more likely to report negative effects from cannabis oils or softgels.
Covering October 17, 2018 to December 31, 2019 and January 1, 2020 to December 31, 2020 there were a total of 378 adverse reactions associated with cannabis products reported to Health Canada’s Canada Vigilance Database.
While the first year of data collection for 2018-2019 showed 219 adverse reaction reports, the following year saw only 159.
In both years, most reports involved legal cannabis products, while others were associated with the illicit market or from an undetermined source, or from mixing other substances with cannabis.
Of these, 198 (77 in the first year and 121 in the second year) were considered “serious,” with hospitalization as the most frequently reported reason for seriousness. The majority of cases in both years originated from consumers and were reported to Health Canada by cannabis licence holders (federal producers). Only 24% of cases in the first year and 11% in the second year were reported by health care practitioners (HCPs).
Health Canada defines an adverse reaction as “a noxious and unintended response to a cannabis product” and defines a serious adverse reaction as “a noxious and unintended response to a cannabis product that requires inpatient hospitalization or a prolongation of existing hospitalization, causes congenital malformation, results in persistent or significant disability or incapacity, is life-threatening, or results in death.”
As the reporting of adverse reactions by consumers, health care practitioners (HCPs), medical cannabis clinics, and retailers is voluntary, Health Canada notes that serious and non-serious cases from these sources are “likely underreported.”
Cannabis licence holders are required by federal regulations to report any serious adverse reactions.
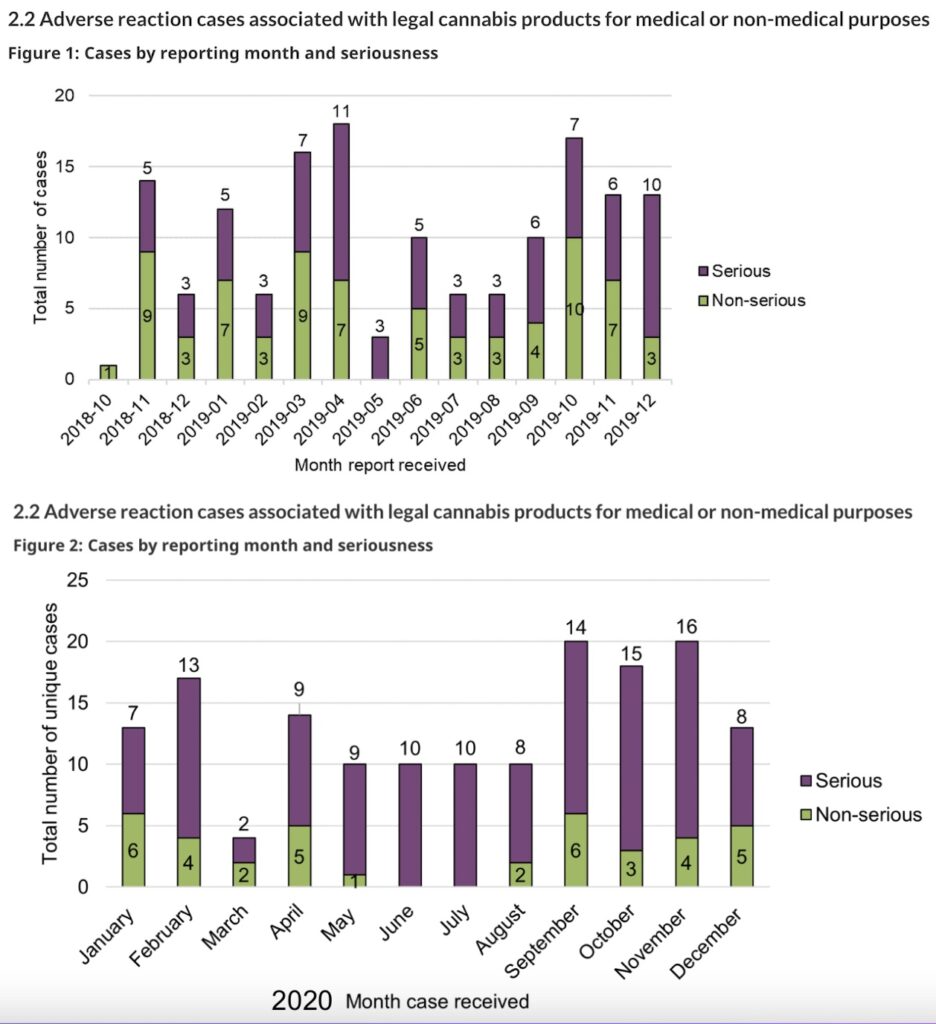
While the total number of serious cases reported to Health Canada increased by 57% between the reporting periods, from 77 cases in 2018–2019 to 121 cases in 2020, cases where hospitalization was required declined, from 43 in the first year to 33 in the second. In the first year, there were seven reports considered life-threatening, while only one was reported as life-threatening in the second year.
In contrast, the total number of non-serious adverse reaction reports decreased by 49%, from 74 in 2018–2019 to 38 in 2020.
However, reports associated with other medically important conditions increased in the second year, from 27 to 86. “Other medically important condition” includes events that are not immediately life-threatening or do not result in death or hospitalization but may jeopardize the patient or may require intervention (for example, ambulatory services, emergency department visits, outpatient visits with an HCP or at-home medical interventions) to prevent a serious outcome.
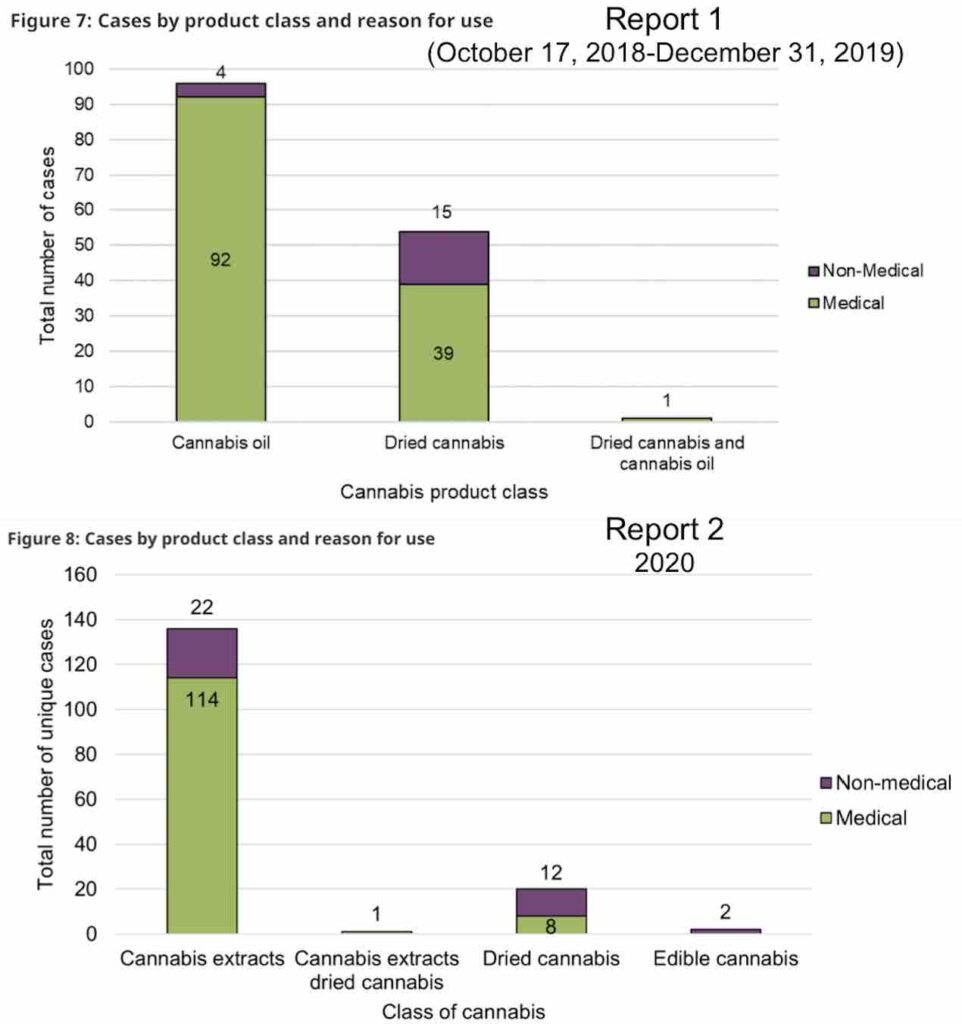
In both years, cases involving legal cannabis products were more likely to involve female adults, and the majority of the time involved cannabis oils or softgels. Very few cases were associated with minors (two in the first year and three in the second).
While the first year’s report did not capture a significant amount of cannabis extracts or edibles (most weren’t yet on the market), in 2020, there was one suspected case of vaping-associated lung illness (VALI) that was reported as involving legal cannabis products, and two suspected cases that were reported as involving undefined cannabis. None of these cases were established to be a confirmed or probable case according to the case definition established by the Public Health Agency of Canada, though.
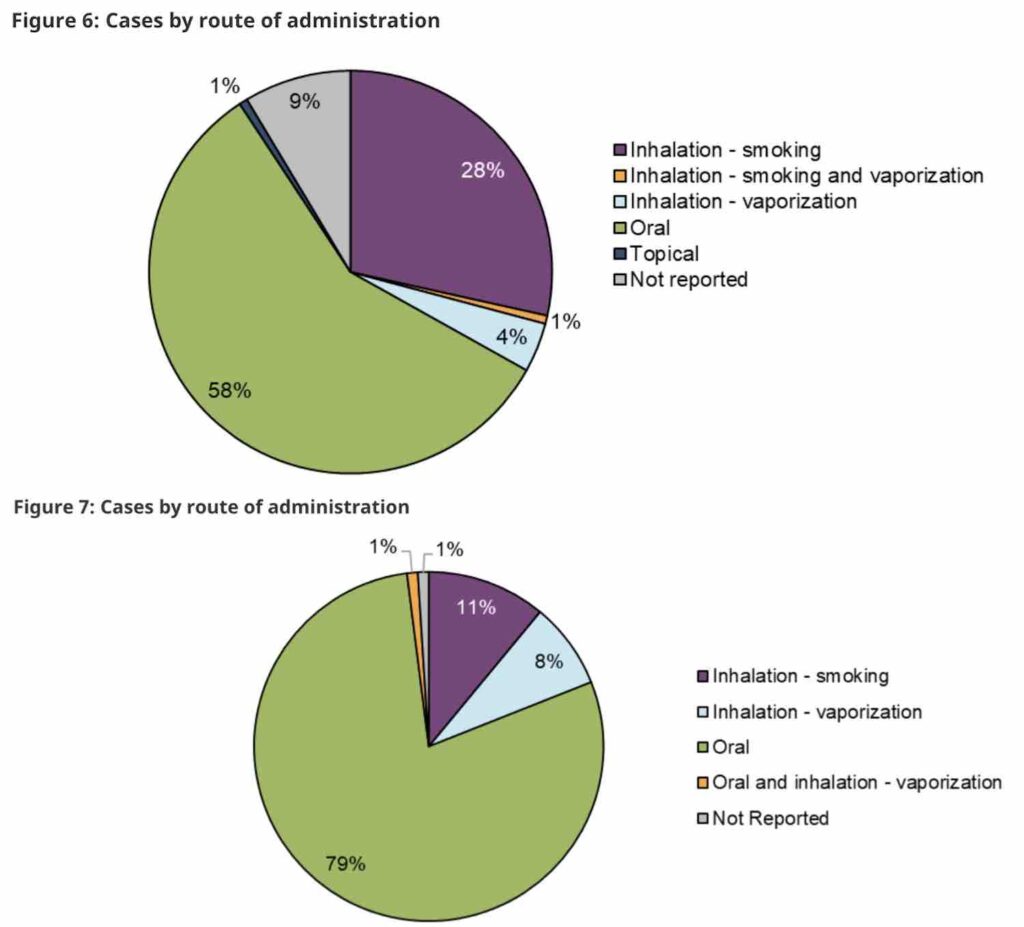
The majority of reports of adverse reactions in both years involved a single legal cannabis product, although some included the mixing of multiple cannabis products. Orally-ingested extracts (ie edibles and/or oils) were the most common product associated with an adverse reaction, followed by inhaled cannabis products.
Cannabis oil products or extracts were more frequently reported in serious cases, while dried cannabis products were more frequently reported in non-serious cases.
Interestingly, most of the cannabis oil products in the adverse reaction cases were considered ‘CBD-dominant’ or ‘CBD-leaning’, while the dried cannabis products were typically reported as ‘THC-dominant’.
Cases involving adults 65 years and older exclusively reported use of cannabis extracts. Cases involving younger adults reported use of dried cannabis and cannabis extracts.
Only those between the ages of 18–64 were involved in adverse reaction cases with vaping liquids, while older adults (≥65 years) were more frequently involved in adverse reaction cases with ingestible oils in liquid form and softgel capsules.
The most common associated events leading to hospitalization were nervous system disorders and psychiatric disorders, followed by “general disorders” and gastrointestinal issues.
The most commonly reported symptoms in the first year were headache, nausea, hallucination, dizziness, and anxiety. Reports of headache and dyspnoea (difficulty breathing) were more frequently associated with THC-dominant products, while reports of dizziness and diarrhea were more frequently reported with CBD-dominant or CBD-leaning products.
In the second year, headaches were replaced by hallucinations as the most frequently reported medical events, followed by dizziness, nausea, euphoria, abnormal feelings, and insomnia.
Different medical events were also associated with different types of products, cannabinoids, and modes of consumption.
In the second year, insomnia and pain were more frequently reported with THC-dominant or leaning products, whereas anxiety and diarrhea were more frequently reported with CBD-dominant or leaning products. Dizziness, loss of consciousness, syncope, and hallucination were also more frequently reported with CBD-dominant or leaning products.
In the first year, instances of headache and dyspnoea (difficulty breathing) were more frequently observed with THC-dominant products, whereas events of dizziness and diarrhea were more frequently reported with CBD-dominant or CBD-leaning products.
Health Canada also notes that more data over the coming years will be needed to draw more solid conclusions. The regulator also highlights that other factors may be contributing to these events including: the age and health status of patients (including pre-existing health conditions and use of concomitant medications); prior exposure to cannabis (for example, cannabis naïve consumers); dosage; route of administration; and knowledge or awareness of effects of cannabis and cannabinoids.
Health Canada also covered one new data point that involved an increased bleeding risk associated with an interaction between orally ingested CBD-dominant cannabis oil products and the anticoagulant medication Warfarin.
Helping to gather data on the topic, the most recent Canadian Cannabis Survey, released in December, also included questions about any accidental exposures to cannabis in the household for humans or pets in the past year.
Under federal cannabis regulations, licence holders are required to submit serious adverse reaction reports for instances involving a cannabis product and are encouraged to voluntarily submit non-serious adverse reaction reports involving a cannabis product. Licence holders can find more information in the Cannabis adverse reaction reporting guide.
Consumers and HCPs are also encouraged to report all adverse reactions to a cannabis product directly to the Controlled Substances and Cannabis Branch (CSCB). Consumers and HCPs may also send a report to the LH of the cannabis product.
That online Cannabis Reporting Form can be found here and includes reports for issues relating to marketing and promotion, products and packaging, as well as potential negative health events.











