A recent publication in Current Opinion in Food Science explores the opportunities and challenges in developing cannabis edibles.
It is widely acknowledged that smoking of cannabis flower is still regarded as the traditional method of use, even though it has obvious public health concerns similar to those observed when smoking tobacco. Alternative methods of consumption include orally administered edibles, which will undoubtedly increase in popularity in jurisdictions where recreational cannabis is legal. However, a rigorous study of the pharmacokinetics of tetrahydrocannabinol (THC) and cannabidiol (CBD) in different edible food matrices has not been conducted at this point.
The potential increase in edibles consumption in Canada poses serious challenges to policy makers because limited research on evaluating how ingestion of THC and CBD, within a food matrix, affects dose delivery, bioactive effects (i.e. potency) and health and safety.
Maximizing bioavailability of cannabis-infused edibles is already a top priority in the cannabis industry and technologies are being adapted from other lipophilic bioactive compounds. Similar systematic research is of utmost importance for THC and CBD edibles to provide knowledge to producers, policy makers and the general public.
Potency is dependent on bioavailability
The primary impediment to effective legislation of cannabis infused-edibles/beverages is the fact that concentration and dose of THC and CBD in the product will not reflect the potency, because potency is dependent on bioavailability. Bioavailability describes the fraction of the administered dose that is able to reach systemic circulation delivering therapeutic effects. The bioavailability of smoked THC is approximately 25% (Huestis and Smith 2014) compared to only 3-6% following the oral ingestion of a 20 mg THC chocolate cookie (Ohlsson, Lindgren et al. 1980).
In another study, THC dissolved in sesame oil showed improved oral bioavailability between 10-20% (Wall, Sadler et al. 1983). This 566% variance (3-20%) in oral bioavailability of THC highlights the impact of the food matrix on THC absorption which leads to unpredictable psychotropic effects in the end user.
Smoking allows the user the ability to self-titrate the dose since the psychotropic effects occur rapidly (i.e., < 10 min) allowing the user to effectively gauge the frequency and amount of cannabis pyrolyzed. Edibles have significantly delayed onset times and the delivery of THC and CBD are highly dependent on the food matrix/delivery vehicle making self-titration much more problematic. To put plainly, reporting THC and CBD levels in edibles will be insufficient for consumers to predict their potency.
Unpredictability concern to public health
This unpredictability is of concern to public health when considering the increased use of edibles, especially in first time users. Further complicating the matter, strict laws are being imposed to regulate blood levels of THC to 2.0 ng/ml while driving. The primary challenge is that bioavailability varies widely depending on formulation of the carrier oil, presence, absence and type of surfactants employed, amount of oil, droplet size of the emulsion, etc.
This will be the first time the government regulates a recreational narcotic where the degree of intoxication depends on the delivery system/edible formulation. The dependence of the THC bioavailability in edibles WILL depend on formulation, unlike tobacco and alcohol.
This will be the first time the government regulates a recreational narcotic where the degree of intoxication depends on the delivery system/edible formulation. The dependence of the THC bioavailability in edibles WILL depend on formulation, unlike tobacco and alcohol.
Maximizing bioavailability priority in cannabis industry
Maximizing bioavailability of cannabis-infused edibles is already a top priority in the cannabis industry and technologies are being adapted from other lipophilic bioactive compounds. Similar systematic research is of utmost importance for THC and CBD edibles to provide knowledge to producers, policy makers and the general public.
The impact of this research will benefit all stakeholders, allowing producers to formulate products with predictable potency, policy makers to establish proper guidelines and limitations and the public will benefit from a clearer messaging on the effects of edible products prior to ingestion.
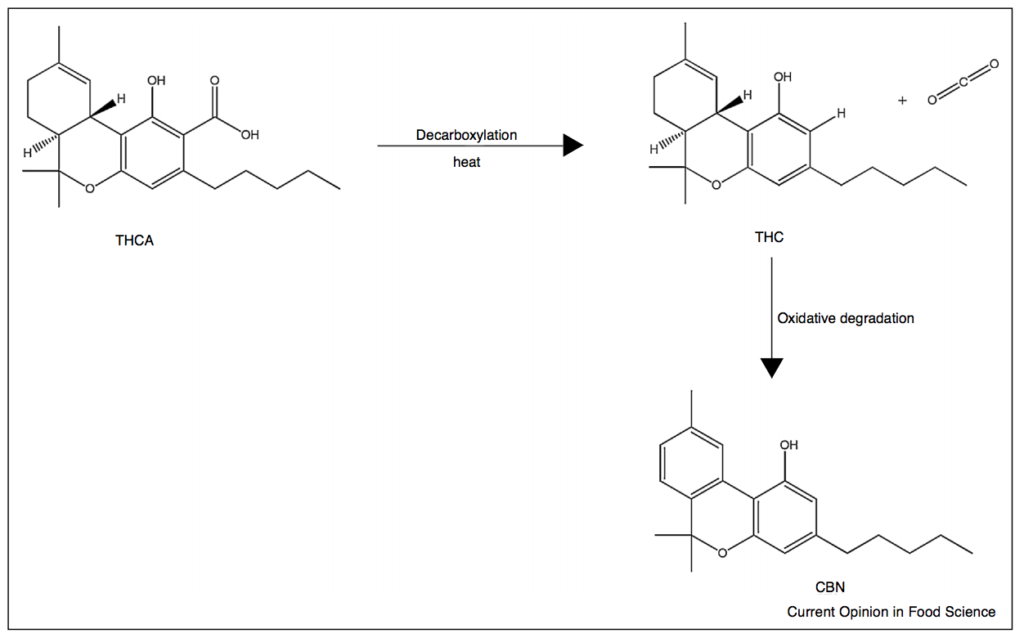
Concurrent with many other aromatic terpenoids, THC is poorly water solubility (i.e., 0.0028 mg/mL). In order to formulate cannabis into edible products for oral administration, the decarboxylated cannabis flower is first extracted.
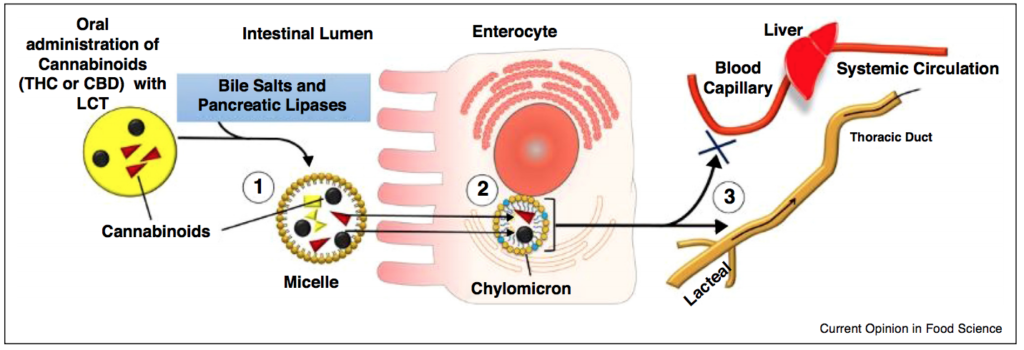
Cannabis extracts have a resinous, oil-like consistency and can be dissolved in organic solvents, lipids and alcohols. An edible oil is produced by simply dissolving the extract in medium-chain triglycerides (MCT) or long-chain triglycerides (LCT). Cannabis oils are currently permissible under the Access to Cannabis for Medical Purposes Regulations (ACMPR) in Canada. Oral co-administration of THC with LCT enhances the systemic exposure of THC and CBD in rats (Zgair, Wong et al. 2016).
The composition and nature of the carrier oil has a substantial role in modulating emulsion bioavailability during simulated gastrointestinal digestion. MCT emulsions have greater rates and extents of lipid solubility compared to LCT emulsions (Yang and McClements 2013). However, greater solubilisation of mixed micelles formed from LCTs leads to higher bioavailability of bioactives following digestion (Yang and McClements 2013). LCTs are used by the pharmaceutical industry to form mixed micelles with hydrophobic cores large enough to accommodate potential bioactives (i.e., THC and CBD), leading to potentially greater bioavailability of THC.
Further formulating of cannabis oil is required to increase the solubility of THC and CBD in water. Encapsulating cannabis oils improves its water solubility and reduces its oxidative susceptibility because the encapsulation matrix acts as a barrier to reduce detrimental effects of the external environments.
Formation of emulsions
The formation of emulsions requires the use of emulsifiers/surfactants which may include: polysaccharides, proteins, phospholipids, natural or synthetic surfactants. Selection of the surfactant is dependent on the nature of the emulsion (i.e., water-in-oil vs. oil-in-water), molecular composition of the oil and the ionic strength of the aqueous phase.
Natural biopolymer-based emulsifiers, such as proteins and polysaccharides, have attracted considerable attention due to the desire by the industry to have clean labels. Natural small molecule surfactants such as phospholipids have also been shown to encapsulate low molecular weight compounds enhancing oral bioavailability of poorly soluble drugs.
Without proper, unbiased research in this area, industry will dictate the products they are able to sell to consumers.
Edibles & bioavailability
An ‘edible’ can be as simple as cannabis oil, produced by dissolving the cannabis extract in carrier oils such as MCT or LCT, or extremely complex where multiple co-surfactants, lipids and viscoelasticity modifiers are added to improve potency. With increasing demand for alternatives to smoking, the industry has already experienced an increase in the number of patents formulating novel cannabis food and beverages.
Current patent literature provides no empirical evidence on bioavailability (i.e., potency) of THC and CBD following ingestion. Furthermore, large companies including Constellation Brands Inc., Molson Coors Brewing Co. and Coca-Cola have either already partnered or are interested in partnering with a licensed cannabis producer to develop cannabis-infused beverages. This is an alarming trend considering empirical evidence regarding the effects of the food matrix on THC and CBD bioavailability has not caught up with industry interests.
Potent formulations may pose greater risk
Without proper, unbiased research in this area, industry will dictate the products they are able to sell to consumers. Industry will benefit when formulated products have increased potency because the associated increase in production cost is negligible. However, more potent edible formulations will pose a greater risk to public health. Research is much needed in this area and will be instrumental in providing information on how structure of food formulations affects the release/absorption of THC and CBD.
This will assist policy makers in designing legislation relating to edibles such as proper labelling, establish guidelines and set limitations on the types of additives, emulsifiers and surfactants permissible to industry. This highlights the dependence of THC and CBD bioavailability on food matrices and may advocate advanced product labeling including both THC and CBD concentrations and also expected bioavailability.
~Peter X. Chen, Ph.D
Peter is the Chief Scientific Officer at Cannaverde Pharma Inc.
Sources:
- Huestis MA and Smith ML: Cannabinoid pharmacokinetics and disposition in alternative matrices. Handbook of Cannabis, Oxford University Press, Oxford 2014: 296-319.
- Ohlsson AJ, Lindgren JE, Wahlen A, Agurell S, Hollister LE and Gillespie HK: Plasma delta‐9‐tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking, Clinical Pharmacology & Therapeutics 1980, 28(3): 409-416.
- Wall ME, Sadler BM, Brine D, Taylor H, and Perez‐Reyes M: Metabolism, disposition, and kinetics of delta‐9‐tetrahydrocannabinol in men and women. Clinical Pharmacology & Therapeutics 1983, 34(3): 352-363.
- Zgair A, Wong JCM, Lee JB, Mistry J, Sivak O, Wasan KM, Henning IM, Barrett DA, Constantinescu CS, Fischer PM, et al.: Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. American Journal of Translational Research 2016, 8(8): 3448-3459.
- Yang Y and McClements DJ: Vitamin E bioavailability: Influence of carrier oil type on digestion and release of emulsified α-tocopherol acetate, Food Chemistry 2013 141(1): 473-481.