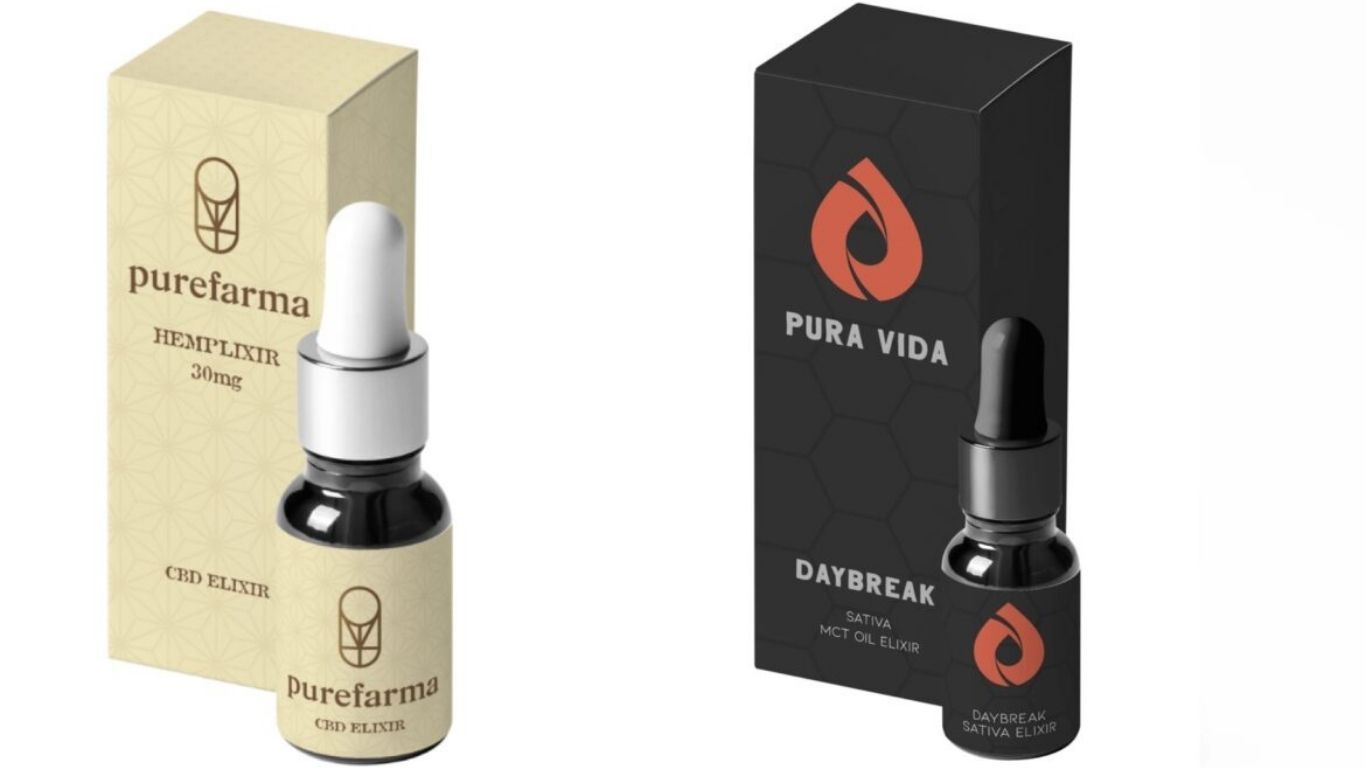
Voyage Cannabis Corp. has voluntarily recalled nine lots of PureFarma and PuraVida cannabis oil due to mislabeling. The were sold through provincially-authorized retailers in British Columbia and to clients registered for medical purposes.
The BC LDB notes they only carried five of those products through their online retail platform and private retail stores.
The incorrect labels state that the product’s intended use is “Inhalation”, instead of “Ingestion”. The immediate container lists the correct intended use.
Health Canada reports that, to date, Voyage Cannabis Corp. has received 3 complaints regarding the labels of the recalled lots and that Health Canada has not received any complaints related to the recalled lots. Neither Health Canada nor Voyage Cannabis Corp. have received any adverse reaction reports for the recalled cannabis product lots.
The following products with the corresponding SKU and lot combinations are eligible for return: PureFarma – Balance 15-15, PureFarma – Hemplixer 15 / Hemplixer 15, PureFarma – Hemplixer 30 / Hemplixer 30, PuraVida – Daybreak Sativa Honey Oil Drops, PuraVida – Nightfall Indica Honey Oil Drops.
8,736 units of recalled product were sold. The recalled product was sold from October 13 to November 5, 2020. Voyage Cannabis is based in BC.